We are proud to report that our biomarker work at Roche has been published:

Synthesis, Characterization, and in vivo Evaluation of a Novel Potent Autotaxin-Inhibitor
Front. Pharmacol., 18 January 2022 | https://doi.org/10.3389/fphar.2021.699535
Autotaxin is an attractive inflammation target because inflammation processes are involved in many diseases with unmet medical needs (neurodegeneration, rheumatoid arthritis, glaucoma, fibrotic processes in various organs). Our LC/MS measurements played a central role in the project’s progress.
Autotaxin converts lyso-phosphatidyl cholines (LPC’s) of various chain lengths to their analogous lysophosphatidic acids (LPA’s) and therefore the levels of the latter are an ideal target for the determination of the enzyme’s activity in-vitro and in-vivo.
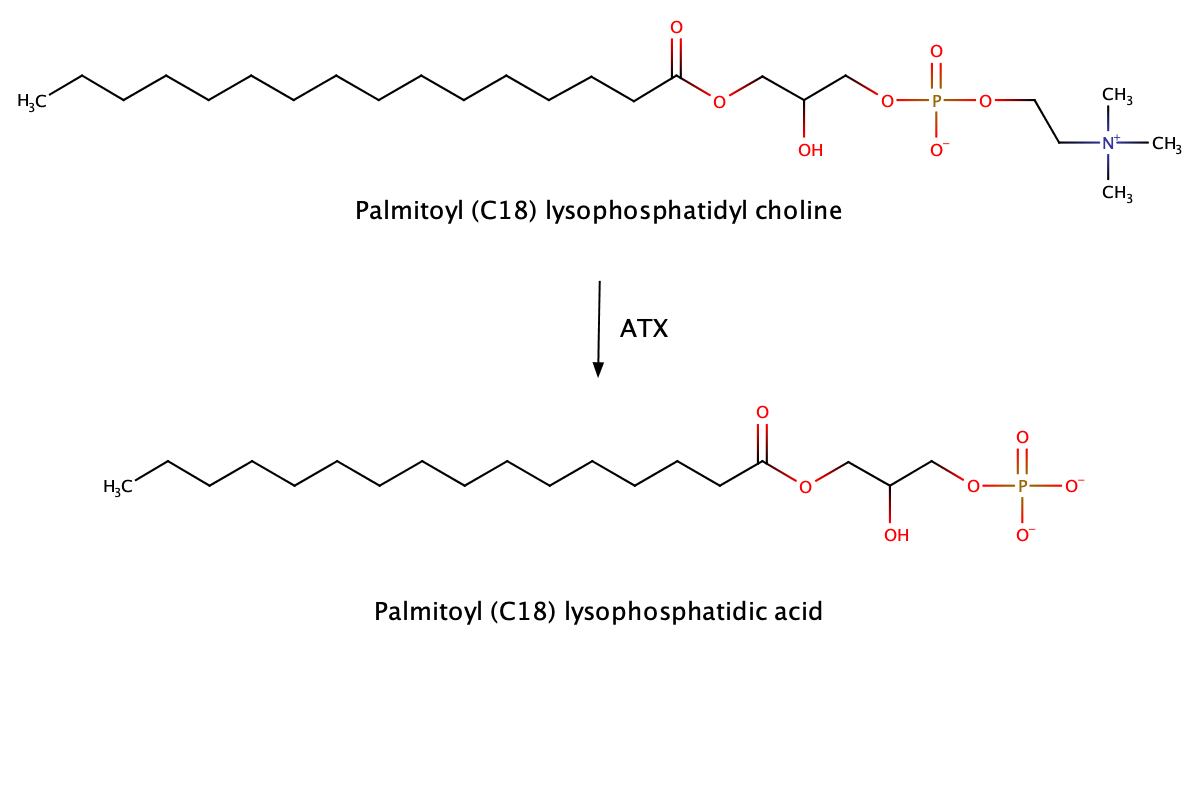
With some HPLC tricks they can be determined by LC/MRM-MS down to the low nanomolar level. Our measurements enabled the ATX activity determination in bioassays, animal studies and patient samples and ensured permanent awareness on the biochemical effect of the candidate drugs, also in cases where the physiological effect was weak. This information is a cornerstone for decisionmaking in project management, and often not available in projects without biomarker strategy.
The difficulty of the measurement was that in most body fluids, very high LPC levels are present, ATX is active and therefore, LPA is produced as soon as temperature rises above 0°C. Sampling, plasma generation and processing has therefore to be done under the coldest possible conditions and in a minimum of time. The successful measurements were obtained by working hand in hand with the in-vivo groups and disease biology.
Figure 2 of the publication shows overlays of the PK and biomarker data that demonstrate a nearly perfect PK-PD relationship.
LC/MS details can be found in the supplementary material under 1.3..
We thank all the involved colleagues, especially V.S., for their continuous support of the project in difficult times, and the project team for the appreciation of our contribution.
